崔永萍课题组报道食管鳞癌中FAT1调控细胞的干性和顺铂耐药性
2022年5月23日山西医科大学转化医学研究中心崔永萍课题组在Molecular and Cellular Biochemistry杂志上发表题为“FAT1 downregulation enhances stemness and cisplatin resistance in esophageal squamous cell carcinoma”的论文,该研究探讨突变基因FAT1对食管鳞癌细胞的干性和顺铂耐药性的影响,为食管鳞癌患者治疗过程中化疗耐药的逆转提供了新的思路和靶点。
食管鳞癌患者由于缺乏早期特异性症状和有效检测手段,首次就诊时多处于中晚期,五年生存率不到30%。治疗手段主要包括手术结合放化疗。化疗过程中产生的药物耐药是导致化疗失败的主要原因,顺铂是食管鳞癌患者的常用化疗药物,顺铂耐药是导致化疗失败的重要因素,因此明确导致顺铂耐药的分子机制尤为重要。
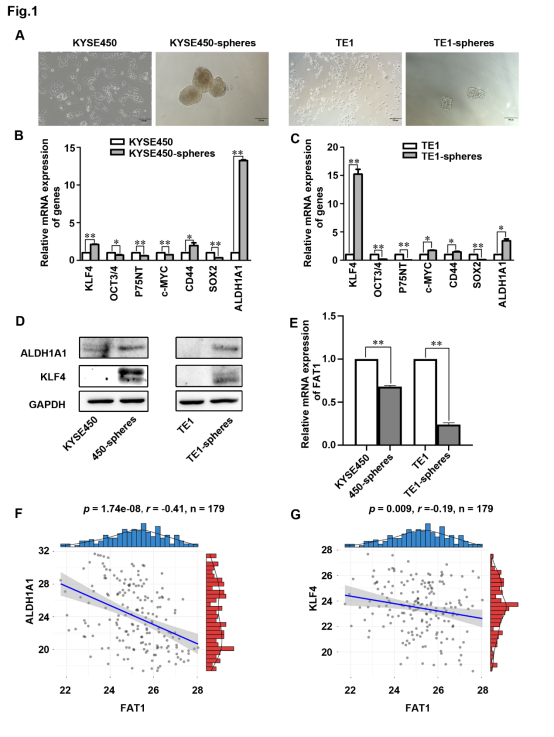
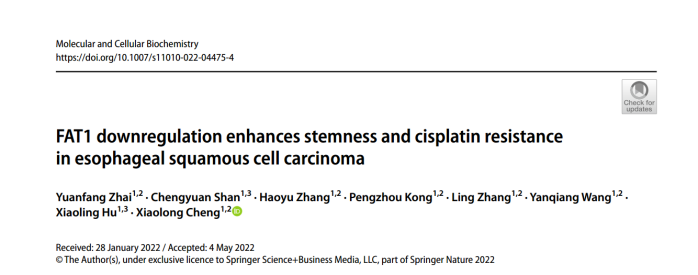
图1.FAT1与食管鳞癌细胞干性的相关性
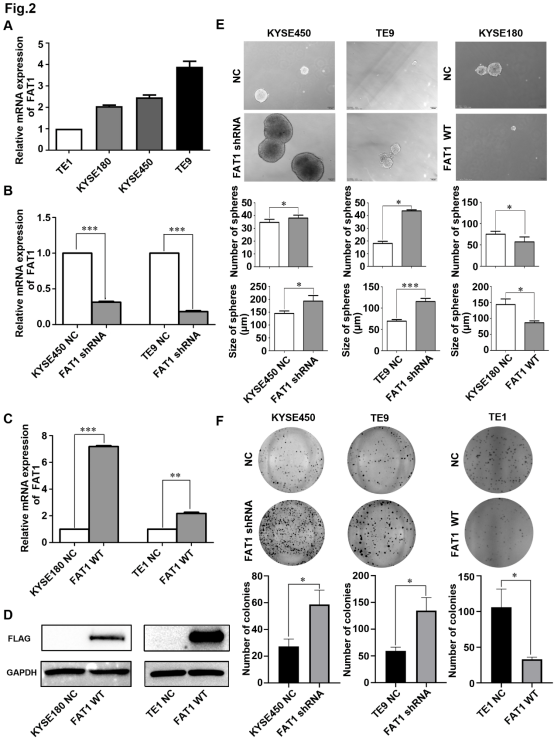 图2.FAT1调控食管鳞癌细胞的克隆和增殖能力
图2.FAT1调控食管鳞癌细胞的克隆和增殖能力
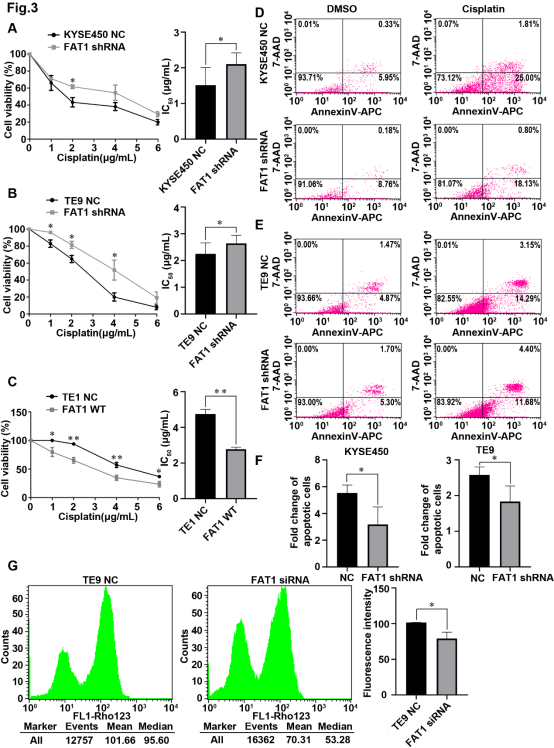 图3.FAT1调控食管鳞癌细胞对顺铂的耐药性
图3.FAT1调控食管鳞癌细胞对顺铂的耐药性
该研究通过探讨FAT1对食管鳞癌细胞干性和顺铂耐药性的调控及其发挥作用的分子机制,明确了FAT1通过Wnt/β-catenin信号通路调控ABCC3进而调控食管鳞癌细胞的干性和耐药性,为食管鳞癌患者顺铂耐药性的逆转提供了新的治疗思路和靶点。
参考文献:
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A(2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. https://doi.org/10.3322/caac.21492
2. Arnold M, Soerjomataram I, Ferlay J, Forman D (2015) Global incidence of oesophageal cancer by histological subtype in 2012.Gut 64:381–387. https://doi.org/10.1136/gutjnl-2014-308124
3. Wang T, Yu J, Liu M, Chen Y, Zhu C, Lu L, Wang M, Min L,Liu X, Zhang X, Gubat JA, Chen Y (2019) The beneft of taxanebased therapies over fuoropyrimidine plus platinum (FP) in the treatment of esophageal cancer: a meta-analysis of clinical studies.
Drug Des Dev Ther 13:539–553. https://doi.org/10.2147/DDDT.S189514
4. Ando N, Kato H, Igaki H, Shinoda M, Ozawa S, Shimizu H, Nakamura T, Yabusaki H, Aoyama N, Kurita A, Ikeda K, Kanda T,Tsujinaka T, Nakamura K, Fukuda H (2012) A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fuorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 19:68–74. https://doi.org/10.1245/s10434-011-2049-9
5. Ter Veer E, Haj Mohammad N, van Valkenhoef G, Ngai LL,Mali RMA, Anderegg MC, van Oijen MGH, van Laarhoven HWM (2016) The efcacy and safety of frst-line chemotherapy in advanced esophagogastric cancer: a network meta-analysis. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djw166
6. Ilson DH (2008) Esophageal cancer chemotherapy: recent advances. Gastrointest Cancer Res 2:85–92
7. Altorki N, Harrison S (2017) What is the role of neoadjuvant chemotherapy, radiation, and adjuvant treatment in resectable esophageal cancer? Ann Cardiothorac Surg 6:167–174. https://doi.org/10.21037/acs.2017.03.16
8. Shibue T, Weinberg RA (2017) EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol14:611–629. https://doi.org/10.1038/nrclinonc.2017.44
9. Farmer P, Bonnefoi H, Anderle P, Cameron D, Wirapati P, BecetteV, Andre S, Piccart M, Campone M, Brain E, Macgrogan G, Petit T, Jassem J, Bibeau F, Blot E, Bogaerts J, Aguet M, Bergh J, Iggo R, Delorenzi M (2009) A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nat Med 15:68–74. https://doi.org/10.1038/nm.1908
10. Scheel C, Eaton EN, Li SH, Chafer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, Weinberg RA (2011) Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 145:926–940. https://doi.
org/10.1016/j.cell.2011.04.029
11. Ni T, Li XY, Lu N, An T, Liu ZP, Fu R, Lv WC, Zhang YW, Xu XJ, Grant Rowe R, Lin YS, Scherer A, Feinberg T, Zheng XQ, Chen BA, Liu XS, Guo QL, Wu ZQ, Weiss SJ (2016) Snail1-dependent p53 repression regulates expansion and activity of
tumour-initiating cells in breast cancer. Nat Cell Biol 18:1221–1232. https://doi.org/10.1038/ncb3425