第一临床医学院郭军红课题组联合第三军医大学王延江教授课题组提出阿尔茨海默病体液生物标志物的新观点
2022年3月19日,来自山西医科大学第一临床医学院的郭军红教授及黄珊博士研究生联合第三军医大学的王延江教授在Neuroscience Bulletin(IF=5.203)上发表了题为“Biofluid biomarkers of Alzheimer’s disease: progress, problems, and perspectives.”的最新文章。该文章围绕AD外周体液生物标志物,首先介绍了A-T-N-X框架的建立过程及过去普遍存在的问题。针对A-T-N-X框架的具体内容进行了深入解读,剖析其中存在的优势及挑战,并系统总结了相应的解决方案,为AD生物标志物的进一步研究探索提供了方向。最后,基于目前的生物标志物检测手段及研究现状,作者提出了更为全面的AD诊断体系,同时也为今后AD及其他神经退行性疾病生物标志物的研究与应用开辟了新的方向。
阿尔茨海默病(Alzheimer’s disease, AD)是一种进行性神经退行性疾病,是最常见的痴呆症类型,以β-淀粉样蛋白(Aβ)斑块形成、细胞内Tau蛋白聚集、神经元和突触丢失等病理表现为特征。随着人口老龄化的进展,AD发病率逐年增加,给社会带来巨大的经济负担。目前,对AD的诊断仍存在困难,生物标志物对于准确和早期识别AD至关重要,也是有效治疗该病的先决条件。
AD生物标志物A/T/N框架在2016年由Clifford等人首次提出,2018年被国际老龄化和阿尔茨海默病协会接受并推广。“A”是指Aβ生物标志物(淀粉样蛋白PET或脑脊液Aβ-42),“T”是Tau生物标志物(脑脊液磷酸化Tau(p-tau)或p-Tau PET),“N”是神经变性或神经元损伤的生物标志物([18F]-氟脱氧葡萄糖 PET、结构磁共振或脑脊液总Tau(t-tau))。这一临床生物学框架描绘了AD的病理生理特征,增加了AD诊断的准确性。然而,现有的框架很难对AD的病理改变提供全面的解释,许多重要的神经损伤标志物未被包含其中。同时,脑脊液检查的有创性及PET扫描价格昂贵且辐射暴露的特点限制了该诊断框架的应用。因此,AD外周生物标志物框架的完善至关重要。
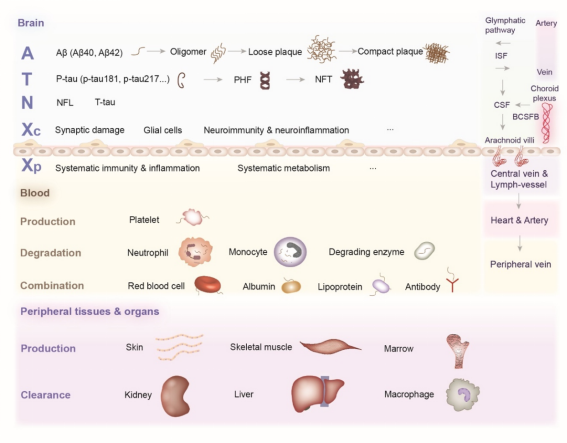
外周体液A-T-N-X框架的建立
由于脑脊液检查具有有创性,同时PET扫描技术价格昂贵且具有辐射暴露,因此,外周生物标志物的研究对AD诊断具有重要价值。目前研究人员发现一些血浆生物标志物,在诊断AD的特异性和灵敏性与脑脊液检查和PET扫描相当。然而,血浆生物标志物的推广存在巨大的困难。①只有来自中枢神经系统(CNS)的一小部分生物标志物能够通过血脑屏障(BBB)、蛛网膜颗粒、类淋巴系统和血管系统进入外周体液系统,进而在血液中被稀释,并且在复杂的血液背景中,生物标志物可以被酶降解,或与各种血液蛋白或血细胞形成复合体,这些因素阻碍了生物标志物的准确检测;②肝脏和肾脏以及相关器官的巨噬细胞可以清除生物标志物,一些外周组织可能会产生相同的生物标志物并释放到血液中;③由于新陈代谢、饮食和药物等因素的不同,外周生物标志物的水平在个体之间波动,同时生物标志物水平随不同疾病的不同时期也会存在波动。所有这些因素都阻碍了血浆生物标志物与大脑中对应的生物标志物之间的相关性。针对这些存在的问题有一些解决方案。首先,开发超敏感技术可以扩大血浆生物标志物的检测范围。同时,新开发的抗体在捕获生物标志物方面更具特异性和敏感性。在血液中浓缩生物标志物的样本处理方法也可以解决稀释效应。其次,血液神经元源性外泌体(NDEs)是从CNS特异性衍生出来的,故NDEs可较为特异性反应CNS中生物标志物水平,同时,NDEs中的生物标志物可以减少血液中的干扰,保护内容物不被降解。第三,不同的采血位置可能会影响检测结果。例如,颈内静脉可能是削弱外周器官清除和血液稀释效果的最佳采血点。第四,BBB紊乱在AD中很常见,其严重程度因疾病分期和个体因素而异。用统一的方法评价BBB通透性有助于更准确地分析外周A-T-N-X系统。除了BBB外,生物标志物从CNS到外周体液的途径仍不完全清楚,需要进一步研究探讨。最后,对AD患者的体液采集方法、采集时间和血浆生物标志物检测方法进行统一能够有效解决个体及时相差异性的问题。
除血浆生物标志物外,许多其他外周生物标志物也在积极研究中,为AD的诊断及筛查提供更多可能性。
A-T-N-X框架的内容
Aβ
Aβ是一种由36~43个氨基酸组成的多肽,由淀粉样前体蛋白(APP)通过β-分泌酶和γ-分泌酶剪切而来。Aβ是AD的中枢生物标志物,同时也是淀粉样斑块的主要成分。脑脊液中Aβ40、Aβ42及其比值和PET检测淀粉样蛋白已成为AD诊断的重要手段。为了解决其有创性及高成本的缺陷,研究人员提出血浆Aβ检测。然而,血浆Aβ作为AD生物标志物仍存在困难。首先,Aβ粘性较高,很难向外周转运。其次,由于血液的稀释等因素,目前的检测手段很难准确测定血液中的可溶性Aβ水平。同时,外周也会产生Aβ,且血液中多种蛋白及细胞与Aβ结合可掩盖其抗原表位使其不易被抗体捕获,降低血液Aβ诊断AD的特异性。此外,前述测定NDEs中的Aβ只能反映CNS中细胞内Aβ水平,而AD病理主要体现在细胞外淀粉样斑块的形成,因此,NDEs中的Aβ无法直接反映AD病理特征。这些问题的存在给外周Aβ作为AD生物标志物带来巨大的挑战。为此,首先要探究Aβ外周转运的机制及其影响因素。其次,检测前的预处理能够降低血液中各种因素的干扰,如ELISA检测Aβ水平前,对血浆样本进行蛋白变性处理,能够使与蛋白结合的Aβ释放出来,提高结果的准确性。同时,AD患者的基因表达在中枢和外周存在差异,有助于区分Aβ的来源,从而帮助准确测定与AD病理相关的外周Aβ水平。
Tau
Tau蛋白是微管相关蛋白Tau(MAPT)基因的产物,具有稳定微管的生理功能。病理性Tau被认为是Aβ的下游蛋白,反映神经元的损伤程度。病理性Tau存在多种翻译后修饰(Post-translational modifications, PTMs),包括磷酸化、乙酰化、甲基化、泛素化、糖基化和硝化等。同时,PTM存在不同的修饰位点,特殊的PTM位点与AD病理相关,有助于AD的诊断。PTM中最常见的是磷酸化Tau,p-Tau是神经纤维缠结的主要成分。其中,脑脊液及血液中的p-tau217、p-tau231和p-tau181对AD的特异性较高。P-tau217、P-tau181等在AD无症状阶段特异性升高,并随着AD的进展而变化,从而有助于AD早期诊断、鉴别诊断、病情变化监测及预后判断。然而,在不同的研究中,p-tau水平对AD诊断的特异性存在差异,这可能与检测前的预处理、检测方法及使用的试剂种类有关。
神经退行性变的生物标志物
神经丝轻链(neurofilament light chain, NFL)作为轴突骨架的组成部分,是反映轴突变性的生物标志物,在临床症状出现前就已出现其水平的改变,同时随着AD的进展发生变化;T-tau是神经变性的生物标志物,反映神经元分泌Tau和皮质厚度的非特异性变化;类视锥蛋白-1(visinin-like protein 1, VILIP-1)是一种在神经元中表达的钙敏感蛋白,反映神经元损伤。然而,这些生物标志物缺乏特异性,在应用时需要与AD特异性生物标志物,如Aβ等联合应用以提高诊断的准确性。
“X”生物标志物
“X”指神经免疫失调、突触功能障碍和BBB改变等其他AD可能发病机制中涉及的生物标志物,在A/T/N框架中加入“X”能够更加全面反映AD病理变化,阐明AD的发病机制。我们将“X”分为两部分,分别是中枢X(XC),即与突触损伤、神经胶质细胞、神经炎症和免疫等相关的生物标记物,和外周X(XP),即与系统免疫、炎症和新陈代谢等相关的生物标记物。
突触功能障碍的生物标志物
突触是学习和记忆的基本结构,突触丢失与认知能力下降有关。反映突触功能障碍的生物标志物有树突状蛋白神经颗粒素(dendritic protein neurogranin, Ng)、突触前蛋白,如神经调节素(neuromodulin, GAP43)、突触体相关蛋白25(Synaptosomalassociated protein 25 , SNAP25)及突触结合蛋白等。
神经胶质细胞、神经免疫和神经炎症的生物标志物
AD的发病与星形胶质细胞和小胶质细胞的激活密切相关,星形胶质细胞和小胶质细胞的生物标志物与AD有关。其中具有代表性的有胶质纤维酸性蛋白(glial fibrillary acidic protein, GFAP)、S100B、几丁质酶-3样蛋白1(chitinase-3-like protein 1, YKL-40)、髓样细胞触发受体2(triggering receptor expressed on myeloid cells-2 , TREM2)及MicroRNA-425等。
系统免疫、炎症和代谢的生物标志物
一些非特异性外周生物标志物,如肿瘤坏死因子、白细胞介素2、免疫球蛋白和补体家族,可用于评估AD的炎症状态。AD常伴随有多种代谢紊乱性疾病,相应的血浆代谢物包括葡萄糖、血脂、氨基酸、维生素和微量元素,都与AD有关。高水平的胆固醇和甘油三酯与AD相关。血液中同型半胱氨酸水平较高,维生素A、B12、C、D、E和叶酸水平较低,与MCI和AD相关。这些生物标志物对诊断AD的特异性不高,但能够与AD特异性生物标志物如Aβ等协同应用提高诊断的准确性及检出率。
AD生物标志物的检测技术
在AD中最广泛使用的生物标志物分析技术是质谱(MS)分析和免疫检测。近来,越来越多的检测技术出现,提高了生物标志物的检测准确性及灵敏性。外周生物标志物的分析基于经典方法或新的超灵敏技术,包括ELISA、单分子阵列(SIMOA)、免疫沉淀/MS、液相色谱-MS、免疫磁性还原(IMR)、多聚体检测系统、还原石墨烯氧化场效应晶体管和冷冻电子显微镜。不同的生物标志物有其合适的检测方法。但是,更可靠、方便、准确的检测手段还需要进一步研究探索。
总结与展望
越来越多的生物标志物运用于AD的临床诊断,提高了AD诊断的准确性,为AD的早期预防及早期诊断提供了可能。然而,我们需要进一步探索更为稳定可靠、特异性高的生物标志物及其检测方法。同时,基于A-T-N-X框架的临床诊断体系也需要进一步完善。我们认为,需要建立以生物标志物为核心,从患者基本信息(包括一般情况)、临床表现(尤其是认知评估)、基础检查及实验室检查、基因检测等多个维度全面评估的AD综合诊断模型。AD的A-T-N-X框架为研究者提供了一种共同语言。今后应更加重视外周生物体液A-T-N-X框架的研究,尤其是利用超灵敏技术提高外周生物体液Aβ检测的准确性。
参考文献
[1] Jia L, Quan M, Fu Y, Zhao T, Li Y, Wei C, Tang Y, Qin Q, Wang F, Qiao Y, Shi S, Wang YJ, Du Y, Zhang J, Zhang J, Luo B, Qu Q, Zhou C, Gauthier S, Jia J; Group for the Project of Dementia Situation in China. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. 2020 Jan;19(1):81-92. doi: 10.1016/S1474-4422(19)30290-X. Epub 2019 Sep 4. PMID: 31494009.
[2] Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, Hampel H, Jagust WJ, Johnson KA, Knopman DS, Petersen RC, Scheltens P, Sperling RA, Dubois B. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016 Aug 2;87(5):539-47. doi: 10.1212/WNL.0000000000002923. Epub 2016 Jul 1. PMID: 27371494; PMCID: PMC4970664.
[3] Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R; Contributors. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018 Apr;14(4):535-562. doi: 10.1016/j.jalz.2018.02.018. PMID: 29653606; PMCID: PMC5958625.
[4] Roberts KF, Elbert DL, Kasten TP, Patterson BW, Sigurdson WC, Connors RE, Ovod V, Munsell LY, Mawuenyega KG, Miller-Thomas MM, Moran CJ, Cross DT 3rd, Derdeyn CP, Bateman RJ. Amyloid-β efflux from the central nervous system into the plasma. Ann Neurol. 2014 Dec;76(6):837-44. doi: 10.1002/ana.24270. Epub 2014 Oct 24. PMID: 25205593; PMCID: PMC4355962.
[5] Liu YH, Wang J, Li QX, Fowler CJ, Zeng F, Deng J, Xu ZQ, Zhou HD, Doecke JD, Villemagne VL, Lim YY, Masters CL, Wang YJ. Association of naturally occurring antibodies to β-amyloid with cognitive decline and cerebral amyloidosis in Alzheimer's disease. Sci Adv. 2021 Jan 1;7(1):eabb0457. doi: 10.1126/sciadv.abb0457. PMID: 33523832; PMCID: PMC7775771.
[6] Cheng Y, Tian DY, Wang YJ. Peripheral clearance of brain-derived Aβ in Alzheimer's disease: pathophysiology and therapeutic perspectives. Transl Neurodegener. 2020 May 7;9(1):16. doi: 10.1186/s40035-020-00195-1. PMID: 32381118; PMCID: PMC7204069.
[7] Wang YR, Wang QH, Zhang T, Liu YH, Yao XQ, Zeng F, Li J, Zhou FY, Wang L, Yan JC, Zhou HD, Wang YJ. Associations Between Hepatic Functions and Plasma Amyloid-Beta Levels-Implications for the Capacity of Liver in Peripheral Amyloid-Beta Clearance. Mol Neurobiol. 2017 Apr;54(3):2338-2344. doi: 10.1007/s12035-016-9826-1. Epub 2016 Mar 9. PMID: 26957302.
[8] Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-beta levels: implications for a diagnostic and therapeutic biomarker. Neurology. 2007 Feb 27;68(9):666-9. doi: 10.1212/01.wnl.0000256043.50901.e3. PMID: 17325273.
[9] Karki HP, Jang Y, Jung J, Oh J. Advances in the development paradigm of biosample-based biosensors for early ultrasensitive detection of alzheimer's disease. J Nanobiotechnology. 2021 Mar 9;19(1):72. doi: 10.1186/s12951-021-00814-7. Erratum in: J Nanobiotechnology. 2021 Apr 26;19(1):118. PMID: 33750392; PMCID: PMC7945670.
[10] Kim K , Lee CH , Park CB . Chemical sensing platforms for detecting trace-level Alzheimer's core biomarkers. Chem Soc Rev. 2020 Aug 7;49(15):5446-5472. doi: 10.1039/d0cs00107d. Epub 2020 Jul 6. PMID: 32627779.
[11] Jia L, Qiu Q, Zhang H, Chu L, Du Y, Zhang J, Zhou C, Liang F, Shi S, Wang S, Qin W, Wang Q, Li F, Wang Q, Li Y, Shen L, Wei Y, Jia J. Concordance between the assessment of Aβ42, T-tau, and P-T181-tau in peripheral blood neuronal-derived exosomes and cerebrospinal fluid. Alzheimers Dement. 2019 Aug;15(8):1071-1080. doi: 10.1016/j.jalz.2019.05.002. PMID: 31422798.
[12] Jia L, Zhu M, Kong C, Pang Y, Zhang H, Qiu Q, Wei C, Tang Y, Wang Q, Li Y, Li T, Li F, Wang Q, Li Y, Wei Y, Jia J. Blood neuro-exosomal synaptic proteins predict Alzheimer's disease at the asymptomatic stage. Alzheimers Dement. 2021 Jan;17(1):49-60. doi: 10.1002/alz.12166. Epub 2020 Aug 10. PMID: 32776690; PMCID: PMC7984076.
[13] Xing W, Gao W, Lv X, Xu X, Zhang Z, Yan J, Mao G, Bu Z. The Diagnostic Value of Exosome-Derived Biomarkers in Alzheimer's Disease and Mild Cognitive Impairment: A Meta-Analysis. Front Aging Neurosci. 2021 Mar 1;13:637218. doi: 10.3389/fnagi.2021.637218. PMID: 33732139; PMCID: PMC7957006.
[14] Pannee J, Gobom J, Shaw LM, Korecka M, Chambers EE, Lame M, Jenkins R, Mylott W, Carrillo MC, Zegers I, Zetterberg H, Blennow K, Portelius E. Round robin test on quantification of amyloid-β 1-42 in cerebrospinal fluid by mass spectrometry. Alzheimers Dement. 2016 Jan;12(1):55-9. doi: 10.1016/j.jalz.2015.06.1890. Epub 2015 Jul 21. PMID: 26206625.
[15] Koychev I, Jansen K, Dette A, Shi L, Holling H. Blood-Based ATN Biomarkers of Alzheimer's Disease: A Meta-Analysis. J Alzheimers Dis. 2021;79(1):177-195. doi: 10.3233/JAD-200900. PMID: 33252080.
[16] Pawlik P, Błochowiak K. The Role of Salivary Biomarkers in the Early Diagnosis of Alzheimer's Disease and Parkinson's Disease. Diagnostics (Basel). 2021 Feb 22;11(2):371. doi: 10.3390/diagnostics11020371. PMID: 33671562; PMCID: PMC7926361.
[17] Yilmaz A, Ugur Z, Bisgin H, Akyol S, Bahado-Singh R, Wilson G, Imam K, Maddens ME, Graham SF. Targeted Metabolic Profiling of Urine Highlights a Potential Biomarker Panel for the Diagnosis of Alzheimer's Disease and Mild Cognitive Impairment: A Pilot Study. Metabolites. 2020 Aug 31;10(9):357. doi: 10.3390/metabo10090357. PMID: 32878308; PMCID: PMC7569858.
[18] Thijssen EH, La Joie R, Wolf A, Strom A, Wang P, Iaccarino L, Bourakova V, Cobigo Y, Heuer H, Spina S, VandeVrede L, Chai X, Proctor NK, Airey DC, Shcherbinin S, Duggan Evans C, Sims JR, Zetterberg H, Blennow K, Karydas AM, Teunissen CE, Kramer JH, Grinberg LT, Seeley WW, Rosen H, Boeve BF, Miller BL, Rabinovici GD, Dage JL, Rojas JC, Boxer AL; Advancing Research and Treatment for Frontotemporal Lobar Degeneration (ARTFL) investigators. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020 Mar;26(3):387-397. doi: 10.1038/s41591-020-0762-2. Epub 2020 Mar 2. PMID: 32123386; PMCID: PMC7101073.
[19] de Wolf F, Ghanbari M, Licher S, McRae-McKee K, Gras L, Weverling GJ, Wermeling P, Sedaghat S, Ikram MK, Waziry R, Koudstaal W, Klap J, Kostense S, Hofman A, Anderson R, Goudsmit J, Ikram MA. Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study. Brain. 2020 Apr 1;143(4):1220-1232. doi: 10.1093/brain/awaa054. PMID: 32206776; PMCID: PMC7174054.
[20] Palmqvist S, Insel PS, Stomrud E, Janelidze S, Zetterberg H, Brix B, Eichenlaub U, Dage JL, Chai X, Blennow K, Mattsson N, Hansson O. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer's disease. EMBO Mol Med. 2019 Dec;11(12):e11170. doi: 10.15252/emmm.201911170. Epub 2019 Nov 11. PMID: 31709776; PMCID: PMC6895602.
[21] Roher AE, Esh CL, Kokjohn TA, Castaño EM, Van Vickle GD, Kalback WM, Patton RL, Luehrs DC, Daugs ID, Kuo YM, Emmerling MR, Soares H, Quinn JF, Kaye J, Connor DJ, Silverberg NB, Adler CH, Seward JD, Beach TG, Sabbagh MN. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer's disease. Alzheimers Dement. 2009 Jan;5(1):18-29. doi: 10.1016/j.jalz.2008.10.004. PMID: 19118806; PMCID: PMC2663406.
[22] Lopatko Lindman K, Weidung B, Olsson J, Josefsson M, Johansson A, Eriksson S, Hallmans G, Elgh F, Lövheim H. Plasma Amyloid-β in Relation to Antibodies Against Herpes Simplex Virus, Cytomegalovirus, and Chlamydophila pneumoniae. J Alzheimers Dis Rep. 2021 Apr 6;5(1):229-235. doi: 10.3233/ADR-210008. PMID: 34113780; PMCID: PMC8150254.
[23] Wang M, Peng IF, Li S, Hu X. Dysregulation of antimicrobial peptide expression distinguishes Alzheimer's disease from normal aging. Aging (Albany NY). 2020 Jan 6;12(1):690-706. doi: 10.18632/aging.102650. Epub 2020 Jan 6. PMID: 31907335; PMCID: PMC6977672.
[24] Kim JW, Byun MS, Lee JH, Yi D, Jeon SY, Sohn BK, Lee JY, Shin SA, Kim YK, Kang KM, Sohn CH, Lee DY; KBASE Research Group. Serum albumin and beta-amyloid deposition in the human brain. Neurology. 2020 Aug 18;95(7):e815-e826. doi: 10.1212/WNL.0000000000010005. Epub 2020 Jul 20. PMID: 32690787; PMCID: PMC7605506.
[25] Inyushin M, Zayas-Santiago A, Rojas L, Kucheryavykh L. On the Role of Platelet-Generated Amyloid Beta Peptides in Certain Amyloidosis Health Complications. Front Immunol. 2020 Oct 2;11:571083. doi: 10.3389/fimmu.2020.571083. PMID: 33123145; PMCID: PMC7567018.
[26] Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, Holtzman DM, Morris JC, Benzinger TLS, Xiong C, Fagan AM, Bateman RJ. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019 Oct 22;93(17):e1647-e1659. doi: 10.1212/WNL.0000000000008081. Epub 2019 Aug 1. PMID: 31371569; PMCID: PMC6946467.
[27] Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Doré V, Fowler C, Li QX, Martins R, Rowe C, Tomita T, Matsuzaki K, Ishii K, Ishii K, Arahata Y, Iwamoto S, Ito K, Tanaka K, Masters CL, Yanagisawa K. High performance plasma amyloid-β biomarkers for Alzheimer's disease. Nature. 2018 Feb 8;554(7691):249-254. doi: 10.1038/nature25456. Epub 2018 Jan 31. PMID: 29420472.
[28] Dujardin S, Commins C, Lathuiliere A, Beerepoot P, Fernandes AR, Kamath TV, De Los Santos MB, Klickstein N, Corjuc DL, Corjuc BT, Dooley PM, Viode A, Oakley DH, Moore BD, Mullin K, Jean-Gilles D, Clark R, Atchison K, Moore R, Chibnik LB, Tanzi RE, Frosch MP, Serrano-Pozo A, Elwood F, Steen JA, Kennedy ME, Hyman BT. Author Correction: Tau molecular diversity contributes to clinical heterogeneity in Alzheimer's disease. Nat Med. 2021 Feb;27(2):356. doi: 10.1038/s41591-021-01251-7. Erratum for: Nat Med. 2020 Aug;26(8):1256-1263. PMID: 33514949; PMCID: PMC8363121.
[29] Barthélemy NR, Horie K, Sato C, Bateman RJ. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer's disease. J Exp Med. 2020 Nov 2;217(11):e20200861. doi: 10.1084/jem.20200861. PMID: 32725127; PMCID: PMC7596823.
[30] Leuzy A, Janelidze S, Mattsson-Carlgren N, Palmqvist S, Jacobs D, Cicognola C, Stomrud E, Vanmechelen E, Dage JL, Hansson O. Comparing the Clinical Utility and Diagnostic Performance of CSF P-Tau181, P-Tau217, and P-Tau231 Assays. Neurology. 2021 Oct 26;97(17):e1681-e1694. doi: 10.1212/WNL.0000000000012727. Epub 2021 Sep 7. PMID: 34493616; PMCID: PMC8605616.
[31] Mielke MM, Frank RD, Dage JL, Jeromin A, Ashton NJ, Blennow K, Karikari TK, Vanmechelen E, Zetterberg H, Algeciras-Schimnich A, Knopman DS, Lowe V, Bu G, Vemuri P, Graff-Radford J, Jack CR Jr, Petersen RC. Comparison of Plasma Phosphorylated Tau Species With Amyloid and Tau Positron Emission Tomography, Neurodegeneration, Vascular Pathology, and Cognitive Outcomes. JAMA Neurol. 2021 Sep 1;78(9):1108-1117. doi: 10.1001/jamaneurol.2021.2293. PMID: 34309632; PMCID: PMC8314178.
[32] Palmqvist S, Janelidze S, Quiroz YT, Zetterberg H, Lopera F, Stomrud E, Su Y, Chen Y, Serrano GE, Leuzy A, Mattsson-Carlgren N, Strandberg O, Smith R, Villegas A, Sepulveda-Falla D, Chai X, Proctor NK, Beach TG, Blennow K, Dage JL, Reiman EM, Hansson O. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA. 2020 Aug 25;324(8):772-781. doi: 10.1001/jama.2020.12134. PMID: 32722745; PMCID: PMC7388060.
[33] Suárez-Calvet M, Karikari TK, Ashton NJ, Lantero Rodríguez J, Milà-Alomà M, Gispert JD, Salvadó G, Minguillon C, Fauria K, Shekari M, Grau-Rivera O, Arenaza-Urquijo EM, Sala-Vila A, Sánchez-Benavides G, González-de-Echávarri JM, Kollmorgen G, Stoops E, Vanmechelen E, Zetterberg H, Blennow K, Molinuevo JL; ALFA Study. Novel tau biomarkers phosphorylated at T181, T217 or T231 rise in the initial stages of the preclinical Alzheimer's continuum when only subtle changes in Aβ pathology are detected. EMBO Mol Med. 2020 Dec 7;12(12):e12921. doi: 10.15252/emmm.202012921. Epub 2020 Nov 10. PMID: 33169916; PMCID: PMC7721364.
[34] Karikari TK, Emeršič A, Vrillon A, Lantero-Rodriguez J, Ashton NJ, Kramberger MG, Dumurgier J, Hourregue C, Čučnik S, Brinkmalm G, Rot U, Zetterberg H, Paquet C, Blennow K. Head-to-head comparison of clinical performance of CSF phospho-tau T181 and T217 biomarkers for Alzheimer's disease diagnosis. Alzheimers Dement. 2021 May;17(5):755-767. doi: 10.1002/alz.12236. Epub 2020 Nov 30. PMID: 33252199; PMCID: PMC8246793.
[35] Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas F, Petzold A, Blennow K, Zetterberg H, Kuhle J. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018 Oct;14(10):577-589. doi: 10.1038/s41582-018-0058-z. PMID: 30171200.
[36] Zetterberg H. Review: Tau in biofluids - relation to pathology, imaging and clinical features. Neuropathol Appl Neurobiol. 2017 Apr;43(3):194-199. doi: 10.1111/nan.12378. PMID: 28054371.
[37] Mielke MM, Przybelski SA, Lesnick TG, Kern S, Zetterberg H, Blennow K, Knopman DS, Graff-Radford J, Petersen RC, Jack CR Jr, Vemuri P. Comparison of CSF neurofilament light chain, neurogranin, and tau to MRI markers. Alzheimers Dement. 2021 May;17(5):801-812. doi: 10.1002/alz.12239. Epub 2021 Mar 4. PMID: 33663022; PMCID: PMC8119371.
[38] Shim KH, Kang MJ, Suh JW, Pyun JM, Ryoo N, Park YH, Youn YC, Jang JW, Jeong JH, Park KW, Choi SH, Suk K, Lee HW, Ko PW, Lee CN, Lim TS, An SSA, Kim S; Alzheimer’s Disease All Markers (ADAM) Research group. CSF total tau/α-synuclein ratio improved the diagnostic performance for Alzheimer's disease as an indicator of tau phosphorylation. Alzheimers Res Ther. 2020 Jul 13;12(1):83. doi: 10.1186/s13195-020-00648-9. PMID: 32660565; PMCID: PMC7359621.
[39] Fink HA, Linskens EJ, Silverman PC, McCarten JR, Hemmy LS, Ouellette JM, Greer NL, Wilt TJ, Butler M. Accuracy of Biomarker Testing for Neuropathologically Defined Alzheimer Disease in Older Adults With Dementia. Ann Intern Med. 2020 May 19;172(10):669-677. doi: 10.7326/M19-3888. Epub 2020 Apr 28. PMID: 32340038.
[40] Groblewska M, Muszyński P, Wojtulewska-Supron A, Kulczyńska-Przybik A, Mroczko B. The Role of Visinin-Like Protein-1 in the Pathophysiology of Alzheimer's Disease. J Alzheimers Dis. 2015;47(1):17-32. doi: 10.3233/JAD-150060. PMID: 26402751.
[41] Zhang H, Ng KP, Therriault J, Kang MS, Pascoal TA, Rosa-Neto P, Gauthier S; Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid phosphorylated tau, visinin-like protein-1, and chitinase-3-like protein 1 in mild cognitive impairment and Alzheimer's disease. Transl Neurodegener. 2018 Sep 10;7:23. doi: 10.1186/s40035-018-0127-7. PMID: 30311914; PMCID: PMC6161434.
[42] Casaletto KB, Elahi FM, Bettcher BM, Neuhaus J, Bendlin BB, Asthana S, Johnson SC, Yaffe K, Carlsson C, Blennow K, Zetterberg H, Kramer JH. Neurogranin, a synaptic protein, is associated with memory independent of Alzheimer biomarkers. Neurology. 2017 Oct 24;89(17):1782-1788. doi: 10.1212/WNL.0000000000004569. Epub 2017 Sep 22. PMID: 28939668; PMCID: PMC5664306.
[43] Öhrfelt A, Dumurgier J, Zetterberg H, Vrillon A, Ashton NJ, Kvartsberg H, Bouaziz-Amar E, Hugon J, Paquet C, Blennow K. Full-length and C-terminal neurogranin in Alzheimer's disease cerebrospinal fluid analyzed by novel ultrasensitive immunoassays. Alzheimers Res Ther. 2020 Dec 22;12(1):168. doi: 10.1186/s13195-020-00748-6. PMID: 33353563; PMCID: PMC7756958.
[44] Tible M, Sandelius Å, Höglund K, Brinkmalm A, Cognat E, Dumurgier J, Zetterberg H, Hugon J, Paquet C, Blennow K. Dissection of synaptic pathways through the CSF biomarkers for predicting Alzheimer disease. Neurology. 2020 Aug 25;95(8):e953-e961. doi: 10.1212/WNL.0000000000010131. Epub 2020 Jun 25. PMID: 32586895.
[45] Sandelius Å, Portelius E, Källén Å, Zetterberg H, Rot U, Olsson B, Toledo JB, Shaw LM, Lee VMY, Irwin DJ, Grossman M, Weintraub D, Chen-Plotkin A, Wolk DA, McCluskey L, Elman L, Kostanjevecki V, Vandijck M, McBride J, Trojanowski JQ, Blennow K. Elevated CSF GAP-43 is Alzheimer's disease specific and associated with tau and amyloid pathology. Alzheimers Dement. 2019 Jan;15(1):55-64. doi: 10.1016/j.jalz.2018.08.006. Epub 2018 Oct 12. PMID: 30321501; PMCID: PMC6333489.
[46] Milà-Alomà M, Brinkmalm A, Ashton NJ, Kvartsberg H, Shekari M, Operto G, Salvadó G, Falcon C, Gispert JD, Vilor-Tejedor N, Arenaza-Urquijo EM, Grau-Rivera O, Sala-Vila A, Sanchez-Benavides G, González-de-Echávarri JM, Minguillon C, Fauria K, Niñerola-Baizán A, Perissinotti A, Kollmorgen G, Suridjan I, Zetterberg H, Molinuevo JL, Blennow K, Suárez-Calvet M; ALFA Study. CSF Synaptic Biomarkers in the Preclinical Stage of Alzheimer Disease and Their Association With MRI and PET: A Cross-sectional Study. Neurology. 2021 Nov 23;97(21):e2065-e2078. doi: 10.1212/WNL.0000000000012853. Epub 2021 Sep 23. PMID: 34556565; PMCID: PMC8610620.
[47] Johnson ECB, Dammer EB, Duong DM, Ping L, Zhou M, Yin L, Higginbotham LA, Guajardo A, White B, Troncoso JC, Thambisetty M, Montine TJ, Lee EB, Trojanowski JQ, Beach TG, Reiman EM, Haroutunian V, Wang M, Schadt E, Zhang B, Dickson DW, Ertekin-Taner N, Golde TE, Petyuk VA, De Jager PL, Bennett DA, Wingo TS, Rangaraju S, Hajjar I, Shulman JM, Lah JJ, Levey AI, Seyfried NT. Large-scale proteomic analysis of Alzheimer's disease brain and cerebrospinal fluid reveals early changes in energy metabolism associated with microglia and astrocyte activation. Nat Med. 2020 May;26(5):769-780. doi: 10.1038/s41591-020-0815-6. Epub 2020 Apr 13. PMID: 32284590; PMCID: PMC7405761.
[48] Carter SF, Herholz K, Rosa-Neto P, Pellerin L, Nordberg A, Zimmer ER. Astrocyte Biomarkers in Alzheimer's Disease. Trends Mol Med. 2019 Feb;25(2):77-95. doi: 10.1016/j.molmed.2018.11.006. Epub 2019 Jan 2. PMID: 30611668.
[49] Bellaver B, Ferrari-Souza JP, Uglione da Ros L, Carter SF, Rodriguez-Vieitez E, Nordberg A, Pellerin L, Rosa-Neto P, Leffa DT, Zimmer ER. Astrocyte Biomarkers in Alzheimer Disease: A Systematic Review and Meta-analysis. Neurology. 2021 May 5:10.1212/WNL.0000000000012109. doi: 10.1212/WNL.0000000000012109. Epub ahead of print. PMID: 33952650.
[50] Llorens F, Thüne K, Tahir W, Kanata E, Diaz-Lucena D, Xanthopoulos K, Kovatsi E, Pleschka C, Garcia-Esparcia P, Schmitz M, Ozbay D, Correia S, Correia Â, Milosevic I, Andréoletti O, Fernández-Borges N, Vorberg IM, Glatzel M, Sklaviadis T, Torres JM, Krasemann S, Sánchez-Valle R, Ferrer I, Zerr I. YKL-40 in the brain and cerebrospinal fluid of neurodegenerative dementias. Mol Neurodegener. 2017 Nov 10;12(1):83. doi: 10.1186/s13024-017-0226-4. PMID: 29126445; PMCID: PMC5681777.
[51] Lee SH, Meilandt WJ, Xie L, Gandham VD, Ngu H, Barck KH, Rezzonico MG, Imperio J, Lalehzadeh G, Huntley MA, Stark KL, Foreman O, Carano RAD, Friedman BA, Sheng M, Easton A, Bohlen CJ, Hansen DV. Trem2 restrains the enhancement of tau accumulation and neurodegeneration by β-amyloid pathology. Neuron. 2021 Apr 21;109(8):1283-1301.e6. doi: 10.1016/j.neuron.2021.02.010. Epub 2021 Mar 5. PMID: 33675684.
[52] Hu YB, Zhang YF, Ren RJ, Dammer EB, Xie XY, Chen SW, Huang Q, Huang WY, Zhang R, Chen HZ, Wang H, Wang G. microRNA-425 loss mediates amyloid plaque microenvironment heterogeneity and promotes neurodegenerative pathologies. Aging Cell. 2021 Oct;20(10):e13454. doi: 10.1111/acel.13454. Epub 2021 Sep 12. PMID: 34510683; PMCID: PMC8520725.
[53] Lai KSP, Liu CS, Rau A, Lanctôt KL, Köhler CA, Pakosh M, Carvalho AF, Herrmann N. Peripheral inflammatory markers in Alzheimer's disease: a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry. 2017 Oct;88(10):876-882. doi: 10.1136/jnnp-2017-316201. Epub 2017 Aug 9. PMID: 28794151.
[54] Hao J, Qiao Y, Li T, Yang J, Song Y, Jia L, Jia J. Investigating Changes in the Serum Inflammatory Factors in Alzheimer's Disease and Their Correlation with Cognitive Function. J Alzheimers Dis. 2021;84(2):835-842. doi: 10.3233/JAD-210552. PMID: 34602472.
[55] Loera-Valencia R, Goikolea J, Parrado-Fernandez C, Merino-Serrais P, Maioli S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer's disease: Potential novel targets for treatment. J Steroid Biochem Mol Biol. 2019 Jun;190:104-114. doi: 10.1016/j.jsbmb.2019.03.003. Epub 2019 Mar 13. PMID: 30878503.
[56] Dimache AM, Șalaru DL, Sascău R, Stătescu C. The Role of High Triglycerides Level in Predicting Cognitive Impairment: A Review of Current Evidence. Nutrients. 2021 Jun 20;13(6):2118. doi: 10.3390/nu13062118. PMID: 34203094; PMCID: PMC8234148.
[57] Lauriola M, D'Onofrio G, Ciccone F, Germano C, Cascavilla L, Paris F, Greco A. Relationship of Homocysteine Plasma Levels with Mild Cognitive Impairment, Alzheimer's Disease, Vascular Dementia, Psychobehavioral, and Functional Complications. J Alzheimers Dis. 2021;82(1):235-248. doi: 10.3233/JAD-210166. PMID: 34057086; PMCID: PMC8293649.
[58] Lopes da Silva S, Vellas B, Elemans S, Luchsinger J, Kamphuis P, Yaffe K, Sijben J, Groenendijk M, Stijnen T. Plasma nutrient status of patients with Alzheimer's disease: Systematic review and meta-analysis. Alzheimers Dement. 2014 Jul;10(4):485-502. doi: 10.1016/j.jalz.2013.05.1771. Epub 2013 Oct 19. PMID: 24144963.
[59] Hampel H, O'Bryant SE, Molinuevo JL, Zetterberg H, Masters CL, Lista S, Kiddle SJ, Batrla R, Blennow K. Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol. 2018 Nov;14(11):639-652. doi: 10.1038/s41582-018-0079-7. PMID: 30297701; PMCID: PMC6211654.
[60] Xu X, Cai X, Zhu Y, He W, Wu Q, Shi X, Fang Y, Pei Z. MFG-E8 inhibits Aβ-induced microglial production of cathelicidin-related antimicrobial peptide: A suitable target against Alzheimer's disease. Cell Immunol. 2018 Sep;331:59-66. doi: 10.1016/j.cellimm.2018.05.008. Epub 2018 May 24. PMID: 29861070.
[61] Lue LF, Kuo YM, Sabbagh M. Advance in Plasma AD Core Biomarker Development: Current Findings from Immunomagnetic Reduction-Based SQUID Technology. Neurol Ther. 2019 Dec;8(Suppl 2):95-111. doi: 10.1007/s40120-019-00167-2. Epub 2019 Dec 12. PMID: 31833027; PMCID: PMC6908530.
[62] Chiu MJ, Chen TF, Hu CJ, Yan SH, Sun Y, Liu BH, Chang YT, Yang CC, Yang SY. Nanoparticle-based immunomagnetic assay of plasma biomarkers for differentiating dementia and prodromal states of Alzheimer's disease - A cross-validation study. Nanomedicine. 2020 Aug;28:102182. doi: 10.1016/j.nano.2020.102182. Epub 2020 Mar 25. PMID: 32222476.
[63] Youn YC, Lee BS, Kim GJ, Ryu JS, Lim K, Lee R, Suh J, Park YH, Pyun JM, Ryu N, Kang MJ, Kim HR, Kang S, An SSA, Kim S. Blood Amyloid-β Oligomerization as a Biomarker of Alzheimer's Disease: A Blinded Validation Study. J Alzheimers Dis. 2020;75(2):493-499. doi: 10.3233/JAD-200061. PMID: 32310175.
[64] Feng L, Huo Z, Xiong J, Li H. Certification of Amyloid-Beta (Aβ) Certified Reference Materials by Amino Acid-Based Isotope Dilution High-Performance Liquid Chromatography Mass Spectrometry and Sulfur-Based High-Performance Liquid Chromatography Isotope Dilution Inductively Coupled Plasma Mass Spectrometry. Anal Chem. 2020 Oct 6;92(19):13229-13237. doi: 10.1021/acs.analchem.0c02381. Epub 2020 Sep 10. PMID: 32847351.
[65] Tarutani A, Miyata H, Nonaka T, Hasegawa K, Yoshida M, Saito Y, Murayama S, Robinson AC, Mann DMA, Tomita T, Hasegawa M. Human tauopathy-derived tau strains determine the substrates recruited for templated amplification. Brain. 2021 Sep 4;144(8):2333-2348. doi: 10.1093/brain/awab091. PMID: 33693528; PMCID: PMC8418341.
[66] Park D, Kim JH, Kim HJ, Lee D, Lee DS, Yoon DS, Hwang KS. Multiplexed femtomolar detection of Alzheimer's disease biomarkers in biofluids using a reduced graphene oxide field-effect transistor. Biosens Bioelectron. 2020 Nov 1;167:112505. doi: 10.1016/j.bios.2020.112505. Epub 2020 Aug 15. PMID: 32841782.